In our last industry profile, we highlighted the Aerospace and Defense (A&D) Industry and mentioned that it is one of the heaviest users of 3D printed materials and components. In today’s blog, we’re covering the medical device industry, which may be the industry that will be most positively impacted by innovation in 3D printing. As for simulation, Ansys and Flownex’s software have been deeply entrenched in the MedDev industry for many years for design and test, as well as meeting the stringent regulations of the industry.
To tell our medical story, we have to look back at our history. As was previously shared, PADT got our start in helping customers in the A&D industry. Due to the many similarities medical devices share with A&D, such as strong quality systems, life or death application, difficult engineering challenges, complex geometries, high-cost components, and more, it was a great area of expansion for PADT early on due to our expertise in addressing customer needs and solving these types of challenges.
PADT offers a variety of products and services to our customers in medical device development. Let’s take a look:
Hardware and Software Products or Medical Device Development
Simulation Software for Medical Device Development
Nearly every product that PADT sells has a strong application in the MedDev industry. Our offering help to design, test and prove functionality, quality, and most importantly, the ability to meet industry requirements for safety. Take our simulation product offerings with Ansys and Flownex – both have been used in medical device design and development for many years.

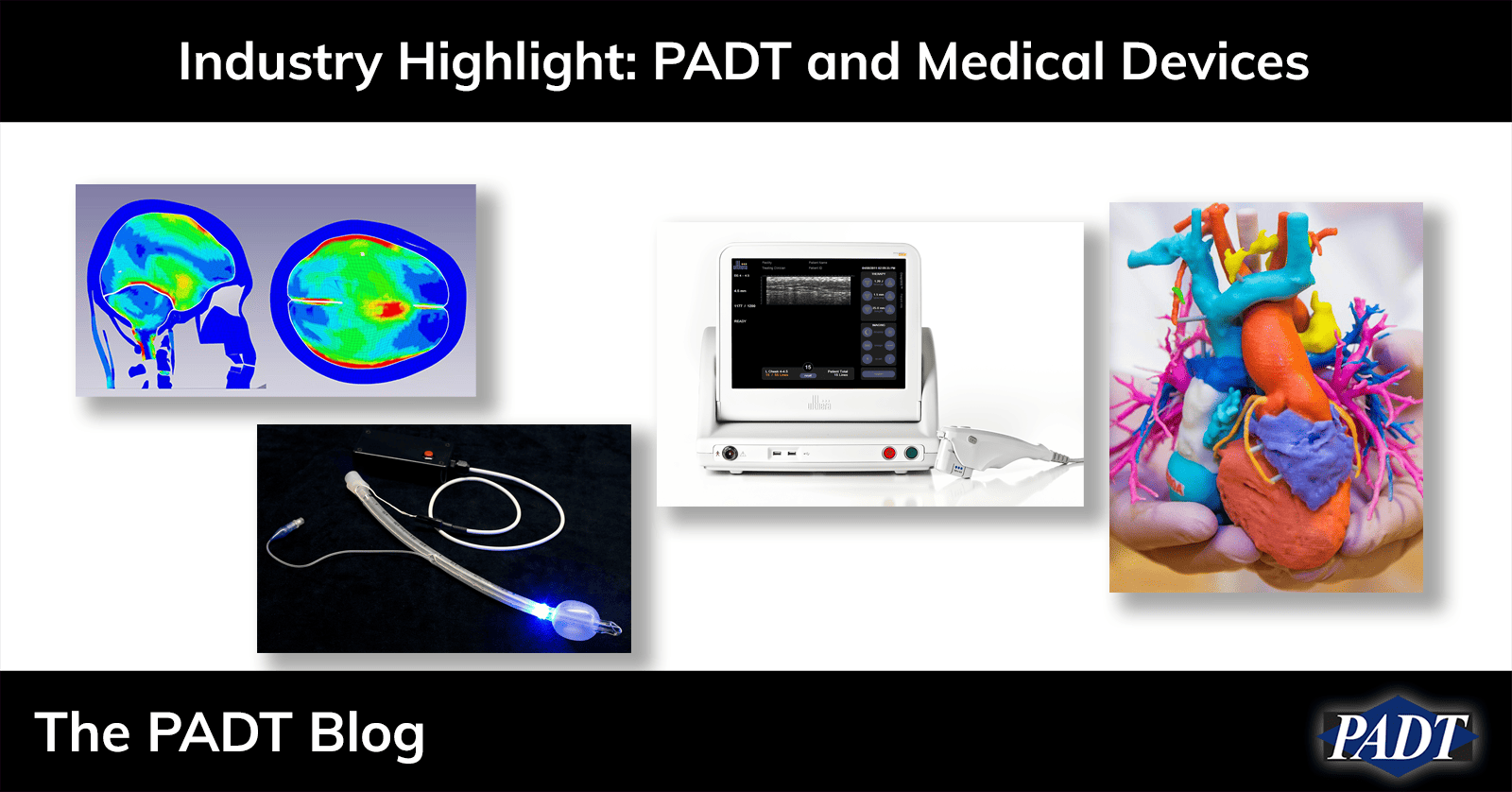
Ansys continues to grow in the medical device industry, recently adding a wide variety of tools designed uniquely for medical use. And because the human body, like most devices, involves multiple physics and are transient, Ansys is the preferred tool for doctors and device designers. One of PADT’s key offerings, specifically for medical, is LS-Dyna for modeling complex device and tissue interaction or HFSS for designing RF therapy devices. This is because the FDA accepts in-silico, simulation-based testing for verification and validation under the ASME V&V 40 standard.
Flownex, on the other hand, is the perfect system modeling tool for managing the fluid flow and thermal behavior of such systems. Many medical devices involve the management of gasses and fluids, including blood. Therefore Flownex can be a great tool for systems involved in blood or sepsis filtration, dialysis, and more for devices that manage oxygen and other gases for patients.
3D Printing for Medical Device Development
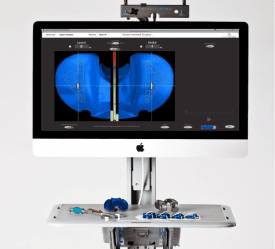
As mentioned above, 3D printing will have an incredible impact in the medical device industry as it continues to grow. Even going as far as having the ability to print human body parts and organs. However, there is still a very key purpose for 3D printing in today’s marketplace. In addition, 3D scanning is another product PADT offers that has many applications in medical.
As a Stratasys reseller for over 20 years, PADT has been providing 3D Printing systems to doctors and hospitals for over two decades. We helped surgeons pioneer using 3D printing for pre-surgical planning or in the operating room as surgical guides. Our customers use the new Stratasys Digital Anatomy printers to get realistic prototypes. We also leverage SLA, FDM, 3P, and Polyjet technologies to prototype and even manufacture their products. Additionally, the new SAF technology is showing promise for manufacturing complex medical devices.

On the metal side, things are starting to get interesting as metal 3D printing manufacturers such as EOS continue to innovate. Metal printing today allows for complex internal geometries, small production runs, and biocompatibility, all possible with the EOS metal laser powder bed fusion solution. As for scanning, PADT also offers optical scanning solutions from GOM and ZEISS. Our customers can bring their devices in-house to scan existing devices, inspect production components, and more to scan bones and even tissue.
Consulting Services for Medical Device Development
When customers need help, especially in the R&D stage, PADT is an excellent resource due to our expertise across industries, and especially in medical device design. Our services range from helping out in test and prototyping, to the full R&D process.
Medical Device Product Development
Our product development team has helped create proof of concept prototypes and assisted in taking a device from concept to manufacturing. We apply tried and true aerospace engineering skills and practices, built-in high-performance quality processes, and flexible project management to medical device R&D to help our customers develop products that meet their product specifications. Our engineers are also all trained on medical device quality systems, including ISO13485, and are adept at fitting into each customer’s system.

Simulation of Medical Devices
As stated above, medical devices operate in a complex, multiphysics environment. PADT’s simulation team are experts at dealing with the material and loading complexities of medical devices. The goal of our simulation team for this industry is to provide actionable information to our customer’s design teams during R&D and then to assist with virtual V&V during the design process.

3D Printing for Medical Device Development and Production

Rounding out our services offerings is a full and complete 3D Printing services team. As of June 2022, PADT offers eight different 3D Printing technologies in-house: SLA, FDM, SLS, P3, LCDP, SAF, Polyjet, and Metal Laser Powder Bed Fusion. Each of these devices runs with multiple materials. And, because PADT supports all these technologies and has engineers that understand medical device development and production, we can provide better prototyping and end-part manufacturing.
Let PADT Bring Dimension to Your Innovations in Medical Devices
Like A&D, medical device development is a tough, complex, and heavily regulated industry. PADT understands those challenges and can help our customers with dedicated medical services and products to get to market faster, with higher quality products. To learn more about our product and service offerings in medical device development, visit our website at www.padtinc.com.