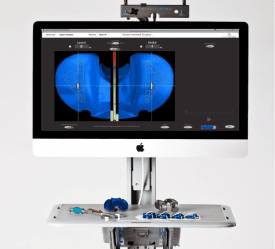
About 40 people joined us at CEI this Monday at the start of Arizona BioScience week for some blunt talk about Medical Device development for startups. It was a great crowd and the questions were almost as (OK, maybe more) useful as the talk.
The gist of the seminar was a look at what it really takes to develop a medical device. We talked about the FDA, ISO 13485, QMS’s and the very well-defined process that all companies must follow. We also talked a bit about transferring to manufacturing and shared some lessons learned.
You can find a PDF of the presentation here:
We look forward to seeing more of you at other AZBio Week events including the AZBio Awards on the 21st and the White Hat Investor conference on the 22nd.
As always, PADT is here to help with your medical device product development, or with the development of any product.